Securing the Future: AI, IoT, and Cybersecurity in Pharma 4.0
- Published:Jan 4, 2024
- ●
- Category:White Paper
- ●
- Topic:Life Sciences
Pharma 4.0 has ushered in a transformative era, in which traditional manufacturing processes have evolved into highly connected and intelligent systems. As advancements such as AI and IoT devices continue to enhance quality and throughput, the need for robust cybersecurity practices has also increased. The security of IoT networks and the integrity of the data they provide are foundational to the success of AI implementation. Without a secure environment, the risk of cyber attacks and data breaches looms, threatening not only the immediate operational stability, but also the public trust and the safeguarding of sensitive personal information.
As we journey through this white paper, we will explore the complexities of cybersecurity in the pharma manufacturing sector, examining the role of AI, IoT, and the urgent need for investment in cybersecurity.
The IoT Revolution in Pharmaceutical Manufacturing
A recent survey of validation experts revealed that an average of 70% of project budgets are currently allocated to Artificial Intelligence (AI) and Machine Learning (ML); an average of 51% to cloud computing; and an average of 47% to IoT implementations. These staggering figures highlight the pharmaceutical industry’s growing investment in advanced technologies. The transition is evident from the changing technological landscape within organizations, where traditional tools like Virtual Private Networks (VPNs) are giving way to Secure Access Service Edge (SASE) models, and firewalls are increasingly cloud-based.
However, adoption of these technologies comes with significant cybersecurity concerns, necessitating a shift towards robust security frameworks like Zero Trust, which assume that no user or device is trustworthy.
The Data Dilemma
Data is the lifeblood of AI models, fueling their learning processes and enhancing their accuracy. However, the primary source of this data is often Internet of Things (IoT) devices, which present a paradox. On one hand, they are prolific data generators, essential for feeding AI systems. On the other hand, they are notoriously insecure due to several factors. The push for low-cost IoT devices frequently results in limited budgets for security, leading to vulnerabilities. Furthermore, there is no formal process for verifying the security of these devices, leaving potential backdoors open. In 2018, it was reported that the Chinese government inserted secret microchips into server hardware to spy on 30 major American companies, including Apple and AWS. Hardware security can never be entirely guaranteed.
Specifically, within pharma, a wide range of technology, including IoT devices, are used to monitor and manage various aspects of drug development and manufacturing. These devices are often interconnected and integral to the manufacturing and data analysis processes, but also pose a risk for potential entry points for cyber attackers. Compromised devices and systems can lead to the theft of intellectual property, disruption of manufacturing processes, and compromise of sensitive patient data.
This security challenge is compounded by a notable labor shortage in cybersecurity. There aren't enough professionals to monitor and secure the vast array of IoT endpoints that constantly generate valuable data. Moreover, when data is collected, the industry faces a dearth of skilled professionals to analyze and process it effectively. This creates a scenario in which AI's assistance is urgently needed to manage and make sense of the data deluge, yet the very tools we rely on for this task remain in a developmental phase, unable to handle the data complexity and volume.
The SolarWinds cyberattack of 2020 serves as a stark reminder of how seemingly secure systems can be vulnerable. This breach of trust resulted in the Federal Response outlined by the US Government Accountability Office.
In 2020, cyber hackers compromised the infrastructure of SolarWinds, a company that produces software for managing and monitoring computer networks for numerous government agencies and private corporations around the world.
In this sophisticated and far-reaching cyberattack, hackers inserted malicious code into the software updates of the SolarWinds Orion platform, allowing them to infiltrate the networks of thousands of SolarWinds customers.
The SolarWinds attack highlighted the vulnerability of interconnected networks and served a wake-up call for all industries, especially those that rely heavily on technology, such as the pharmaceutical industry.
The attack underscores the need for a robust security model that can safeguard pharmaceutical innovation. As excitement builds around greater introduction of AI and IoT, it’s important that these critical organizations continue to prioritize security from the onset, as they advance their technology. The goal should be to create an ecosystem in which data collection, analysis, and the development of AI tools are conducted in a secure, vigilant environment. Zero Trust emerges as a highly effective solution, offering comprehensive protection for IoT devices and networks
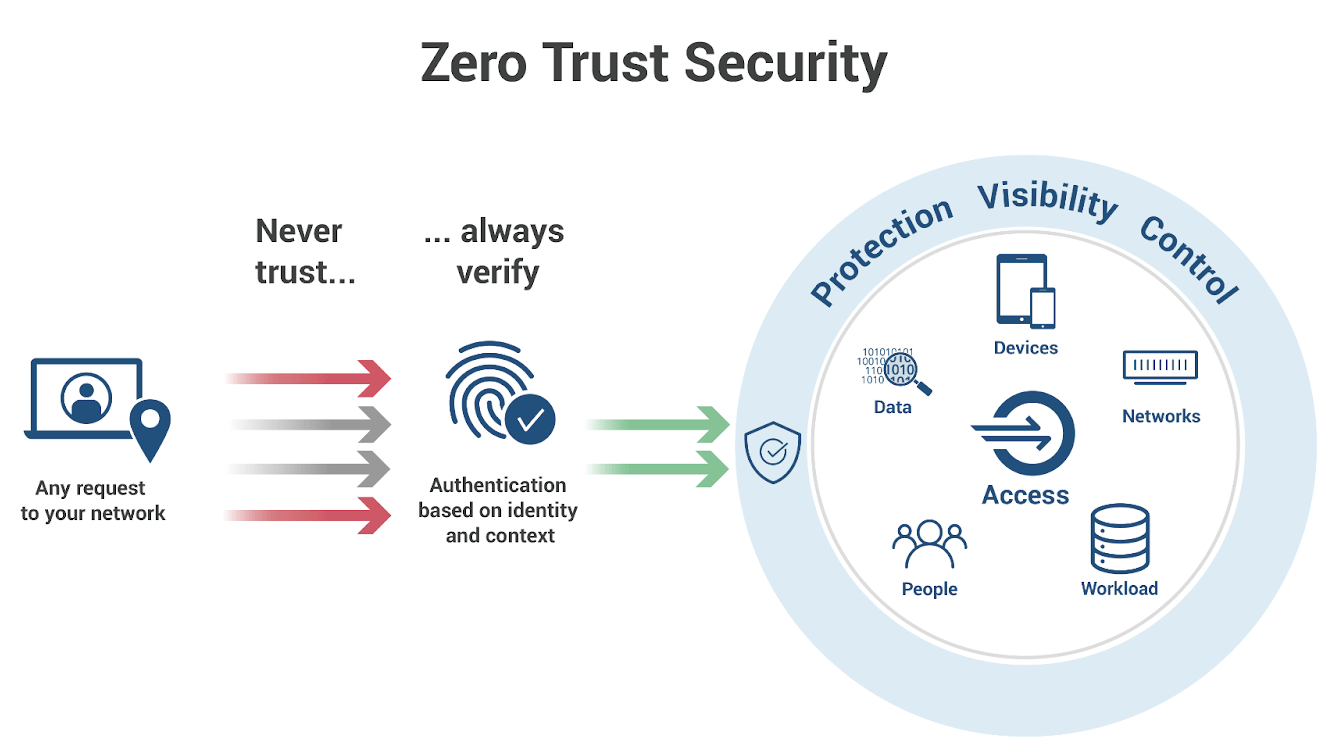
The Case for Zero Trust
As AI and IoT implementations accelerate, the adoption of a Zero Trust security model becomes crucial. Zero Trust operates on the principle of, “never trust, always verify.” This means that no device or user is trusted by default, even if they are inside the network perimeter.
The key components of a Zero Trust model that are particularly relevant for pharma companies include:
- Microsegmentation: Dividing the network into smaller zones to prevent lateral movement of attackers within the network.
- Least Privilege Access: Limiting user access to only what is needed for their specific role, reducing the potential damage from compromised credentials.
- Multi-Factor Authentication (MFA): Requiring multiple forms of verification before granting access.
- Continuous Monitoring: Keeping a vigilant watch on network activities to quickly detect and respond to suspicious actions.
For pharma companies, implementing a Zero Trust architecture could mean a comprehensive overhaul of their current security practices. It requires shifting from a perimeter-based security approach to one that assumes a breach, verifying every request as if it originated from an open network. What’s the benefit of this approach? For pharma, organizations can expect enhanced protection of sensitive data such as drug formulas and patient information; more robust compliance adherence; and resilience against future attacks.
As the industry continues to embrace technological advancements, the adoption of Zero Trust is not just a recommendation, but an immediate necessity for ensuring security while fostering innovation. In 2022, the Department of Defense (DoD) even adopted a Zero Trust approach in order to achieve cyber resiliency, secure enterprise information and data sharing with external partners.
However, implementing Zero Trust is not a "set it and forget it" solution. It requires continuous monitoring and adaptation, particularly as the network evolves and new devices are added.
The GenAI and Specialized AI Cycle
The evolution of AI has been marked by a fascinating cycle, moving from highly specialized applications, to more generalized AI functions, and back toward specialization. AI systems were originally intended to address specific tasks, such as accurately identifying objects in images – a tool for example that could distinguish a horse from other animals in a picture with impressive precision.
As technological advancements progressed, AI applications shifted toward tackling a broader range of tasks, such as analyzing large volumes of data across various fields, unearthing valuable insights that were previously unattainable due to the sheer scale of the data involved.
This transition to general AI has significant implications, particularly in data-intensive industries like pharmaceutical manufacturing. With the ability to process vast datasets, general AI can help identify patterns, predict trends, and even uncover anomalies that specialized AI might miss. This capability is invaluable to managing complex manufacturing processes, ensuring quality control, and enhancing decision-making.
As general AI sifts through massive datasets, it generates more refined and targeted data. This refined data can then be used to develop new, more effective specialized AI systems, tailored to specific challenges within the pharma industry. It creates a feedback loop where general AI aids in creating enhanced specialized AI, which in turn produces more focused data for general AI to process.
In the context of pharmaceutical manufacturing, this cyclical evolution of AI promises a future where both generalized and specialized AI systems work in tandem. General AI will handle the heavy lifting of data processing, while specialized AI will tackle precise tasks, from monitoring specific stages of drug production to ensuring compliance with regulatory standards. This symbiotic relationship between different types of AI will drive innovation, enhance security, and improve efficiency in pharma manufacturing and beyond.
While AI continues to transform the pharma industry, ongoing viruses, spam, and cyberattacks serve as a reminder that while powerful, AI cannot entirely replace human oversight and expertise. In order for AI to reach its full potential it must be transparent, continuously adaptive, and complemented by skilled cybersecurity professionals who can interpret and make actionable insights.
Conclusion
AI and IoT are shaping the future of numerous critical industries, such as pharmaceutical manufacturing. Its potential to transform operations, enhance decision-making, and revolutionize productivity is profound.
For companies in the pharmaceutical industry, the stakes are exceptionally high. Any compromise in cybersecurity can lead to public scrutiny, loss of intellectual property, and a significant setback in their competitive edge. In an industry where innovation is key and the race to develop the next breakthrough drug is relentless, distractions from cyber threats can be costly. Instead of focusing on navigating cyber attacks and mitigating their aftermath, companies need to ensure their environments are secure from the outset. This will enable them to concentrate on what truly matters – advancing the future of pharmaceuticals and contributing to global health and well-being.
As we embrace the potential of AI to revolutionize industries such as pharma, we must equally prioritize our framework for establishing and maintaining robust cybersecurity practices. Only in a secure, well-guarded technological landscape AI can truly flourish and propel pharmaceutical manufacturing forward.
Interested in getting more out of your data using AI tools and smart features that are purpose-built to meet pharma’s needs? Apprentice leverages the latest technology to enhance efficiency and improve quality across the product lifecycle. The Tempo Manufacturing Cloud is engineered to cover a wide array of manufacturing use cases, offering an all-in-one platform that integrates seamlessly across multiple functions to enhance efficiency and improve quality.